云舟生物科技(广州)股份有限公司品牌商
8 年
手机商铺
- NaN
- 0.5
- 1.5
- 0.5
- 3.5
公司新闻/正文
了解EtBr:如何确保我的电泳条带正确对应我的样本?
517 人阅读发布时间:2023-06-05 10:30
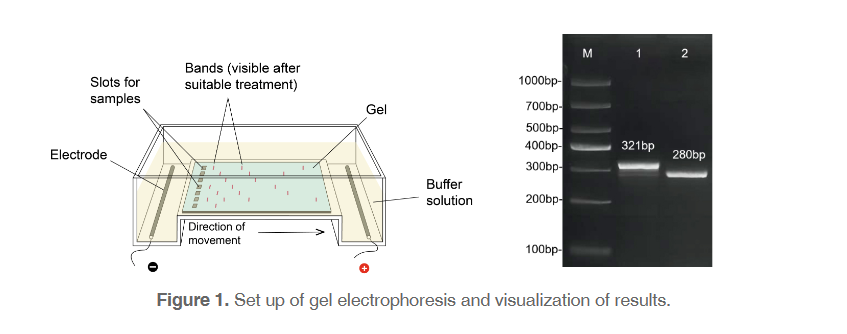
Basics of gel electrophoresis
Since the 1960’s, early in the days of molecular discovery, researchers have utilized the process of electrophoresis, movement under the influence of an electric current, to separate DNA molecules migrating differently. Despite the incredible changes that have occurred in molecular biology labs since then, gel electrophoresis has remained surprisingly unchanged (Figure 1). This is largely due to the technical and theoretical simplicity of the process: DNA, which is negatively-charged, migrates through a solution along an electric current from the negative to the positive side of a chamber (always run to red!). However, the DNA must travel through a gel matrix, typically made up of agarose. Small pieces of DNA travel through the holes in the matrix quickly, while larger pieces of DNA travel more slowly. A gel that is run at 100 V for about 1 hour will generally have large pieces of DNA that have travelled a short distance, and smaller pieces that have travelled a longer distance. Using a reference ladder with bands of known sizes, researchers can confirm that their DNA sample contains the appropriately sized DNA. Bands of DNA can then be excised and recovered for downstream applications.
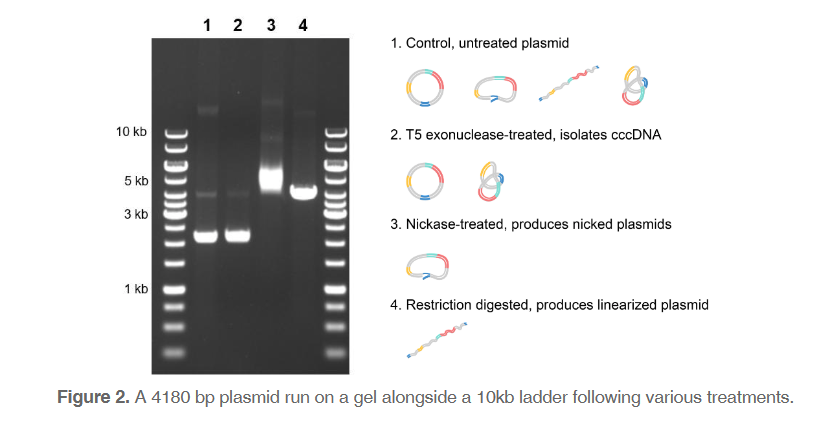
Size can be tricky
One key often-overlooked aspect of gel electrophoresis is the complexity of the “size” of pieces of DNA. Size does not always correlate directly with the length of the DNA in base pairs, particularly when the sample being run is plasmid DNA. A plasmid with the exact same sequence can be in various conformations, resulting in completely different “sizes” and therefore positions on the gel (Figure 2). When the plasmid is partially cut and only one of the strands is broken, this produces nicked plasmids. Nicked plasmids can occur during sample handling by physical tear. In this conformation, the plasmid stays as a full circle and migrates very slowly. Plasmids linearized with restriction enzymes will run through the gel slightly faster, while in-tact circular DNA will move even farther in a given amount of time. However, even within circular DNA (often called covalently closed circular DNA, or cccDNA), there are many possible conformations. The most compacted cccDNA, called supercoiled DNA, can be wound either positively or negatively. Positively supercoiled DNA is slightly smaller than negatively supercoiled, resulting in further migration along the gel.
We can visualize these different conformations within a sample or isolate them through treatments. Untreated sample may contain plasmids in any conformation, but cccDNA may be isolated using T5 exonuclease, which removes nicked and linearized DNA in the sample. The sample can also be treated with nickase, an enzyme that introduces single-strand breaks and produces nicked plasmids. Finally, restriction enzymes can be used to create linearized plasmid.
All about ethidium bromide
Since the 1970’s, DNA samples and reference ladders have often been visualized using ethidium bromide (EtBr). This compound fluoresces strongly under UV light when bound to DNA, allowing visualization on the gel. However, when introducing a compound that binds to your sample, you are introducing a variable that must be considered, particularly for nuanced cases like plasmid DNA. EtBr can affect the conformation of your plasmid, obscuring the perspective on your sample.
EtBr is typically used at concentrations of 0.2-0.5 ug/mL, and while increasing concentrations significantly does not affect bands for smaller plasmids, larger plasmids can exhibit variations in number of bands and positions. This is likely due to EtBr causing greater intercalation within the DNA, “locking” the plasmid into its conformation. For instance, EtBr and its alternative have been shown to alter conformation of supercoiled DNA, resulting in higher proportions of negatively supercoiled DNA.
While concentration of EtBr can impact the display of your sample, any interactions with your DNA before your sample is run can obscure the true picture. We can avoid this issue completely by simply treating gels with EtBr after electrophoresis. Comparing a smaller plasmid that was run on a gel where EtBr was already incorporated (in-gel) to a gel where EtBr was added after electrophoresis (post-electrophoresis), there is little difference when observing linearized and cccDNA (Figure 3A). However, nicked plasmids tend to move farther, likely because of EtBr intercalation tightening the conformation. For larger plasmids, in-gel versus post-electrophoresis staining cause much more significant differences. Both position and number of bands changes for most of the treatments (Figure 3B). Importantly, when comparing treatment 1 (untreated plasmid) to treatment 2 (cccDNA isolation), in-gel staining shows no differences, while post-electrophoresis staining highlights the removal of the nicked DNA from the sample.
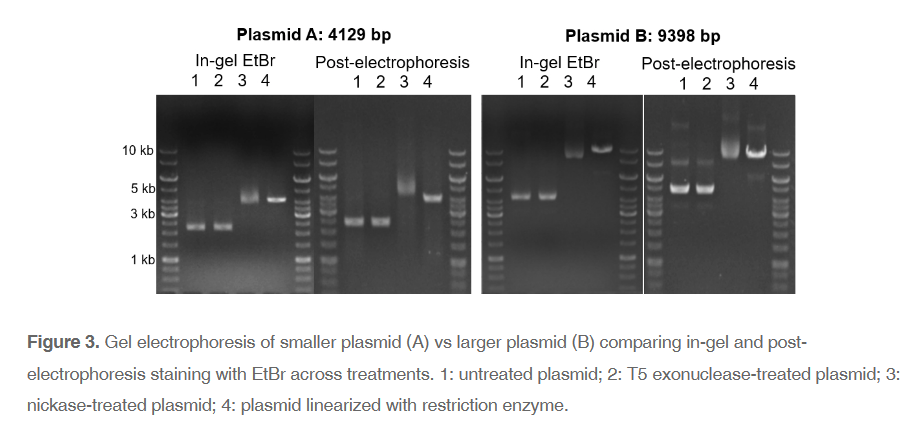
This discrepancy is important to note because the gold-standard for high-quality plasmid is supercoiled DNA, which typically appears as the lowest band on the gel. Note: a faint band of circular single-stranded DNA is occasionally present below the strong supercoiled band. With in-gel staining of plasmids, interpretations of conformation and quality may be biased. The same plasmid and treatment viewed with post-electrophoresis staining, particularly compared to T5 exonuclease treated sample, shows a clear and unbiased view of your sample for quantification as well as quality control. Additionally, comparing in-gel and post-electrophoresis staining highlights how malleable conformation within cccDNA is. Relaxed cccDNA can easily shift to supercoiled, or vice versa. So, focusing on supercoiled DNA rather than cccDNA as a reflection of quality may be placing too high importance on the transient factors interacting with your sample, including EtBr.
Having gel results that accurately represent your sample is important from small-scale cloning experiments to GMP-grade production. If the gel is misread or contains misleading results, downstream applications will be affected. Gel electrophoresis is a foundational tool for numerous molecular biology experiments, so it is important that the foundation is strong and reliable for success.
Sources
Aaij C, Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192-200. doi: 10.1016/0005-2787(72)90426-1. PMID: 5063906.
Mikhailov VS, Okano K, Rohrmann GF. Specificity of the endonuclease activity of the baculovirus alkaline nuclease for single-stranded DNA. J Biol Chem. 2004 Apr 9;279(15):14734-45. doi: 10.1074/jbc.M311658200. Epub 2004 Jan 21. PMID: 14736888.
Sigmon J, Larcom LL. The effect of ethidium bromide on mobility of DNA fragments in agarose gel electrophoresis. Electrophoresis. 1996 Oct;17(10):1524-7. doi: 10.1002/elps.1150171003. PMID: 8957173.
Stellwagen NC. Electrophoresis of DNA in agarose gels, polyacrylamide gels and in free solution. Electrophoresis. 2009 Jun;30 Suppl 1(Suppl 1):S188-95. doi: 10.1002/elps.200900052. PMID: 19517510; PMCID: PMC2757927.
Stettler UH, Weber H, Koller T, Weissmann C. Preparation and characterization of form V DNA, the duplex DNA resulting from association of complementary, circular single-stranded DNA. J Mol Biol. 1979 Jun 15;131(1):21-40. doi: 10.1016/0022-2836(79)90299-7. PMID: 490644.